Protein-Cellular Interfaces
Based on our understanding of the protein-protein interfaces to form a controllable nanostructure to tune biochemical, chemical, mechanical cues, we apply this system to interrogate the protein-cellular interface. Protein-cellular interfaces play a critical role in the development of regenerative medicines and novel therapeutics.
Phage-based nanofiber matrices for regulating cell behavior:
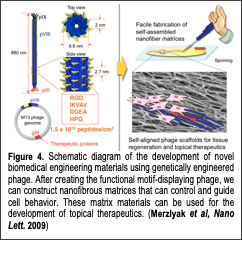
The primary goal of this research is to understand how chemical Text Box: Figure 4. Schematic diagram of the development of novel biomedical engineering materials using genetically engineered phage. After creating the functional motif-displaying phage, we can construct nanofibrous matrices that can control and guide cell behavior. These matrix materials can be used for the development of topical therapeutics. (Merzlyak et al, Nano Lett. 2009)and physical structures affect the interactions between proteins and cells in guiding cellular behavior to develop novel regenerative tissue matrices. Designing biomimetic materials with precisely controlled structural organization that closely mimics the natural tissue environment is critical for the development of regenerative medicines. Many attempts have been made to mimic natural tissue microenvironments in vivo by using an extracted extracellular matrix (ECM) or synthetic ECM-mimetic materials to manipulate the ECM’s biological, chemical and mechanical properties. Although the resulting structures provide great promise for improving cell behavior, emulating complex tissue microenvironments and precisely controlling biochemical and physical cues using conventional approaches are still challenging. Any modification of chemical structures requires labor-intensive chemical synthesis, and the resulting chemical structures affect other important physical and mechanical parameters. A material that mimics the nanofibrous structure of the ECM and whose biochemical structure can be conveniently modified with little change in physical structure and assembly processes would be very desirable for investigating various biochemical cues and their effects on cellular growth processes. Recently, we developed novel phage-based biomimetic nanofiber matrices that could easily be engineered to display various biochemical cues and form self-assembled nanostructures for regulating cellular behaviors (Fig. 4). Using this phage-based tissue matrix system, we investigated various protein and cellular interfaces and discovered new findings on the interactions between protein and cells. In our recent work (Yoo et al, Biomacromolecules 2011), we examined the effects of a collagen-derived biochemical cue (DGEA) on bone cells. We engineered the M13 phage to display the DGEA-peptide in high densities on their major coat proteins and studied their effects on mouse derived bone stem cells (preosteoblasts, MC3T3-E1). Through our study, we verified that the DGEA-peptides could stimulate bone stem cells to outgrow, an event that is linked to osteogenic differentiation. We expanded our protein-cell interface investigation by using other cell types and biochemical cues. Our papers (Merzlyak et al, Nano Letters 2009 and Chung et al, Langmuir 2010, Chung et al, Nature 2011) reported that phages engineered to express RGD (integrin binding peptide) and IKVAV (neural cell stimulating peptide) peptides formed two- and three-dimensional matrices that regulate the directional growth of neural cells in a chemical and physical cue specific manner. In addition, our paper (Yoo et al, Soft Matter 2010) presented a facile strategy for immobilizing growth factors on genetically engineered phage matrices for tissue regeneration. We modified M13 phages to express streptavidin-binding peptides (HPQ) and/or integrin binding peptides (RGD) on their major and minor coat proteins. The resulting phages formed nanofibrous matrices that could easily immobilize the streptavidin-conjugated growth factors FGF-b and NGF for neural cell proliferation and differentiation. We verified that the immobilized growth factors possessed a prolonged ability to stimulate the target cells. We demonstrated the synergistic roles of the growth factors and integrin binding peptides in controlling cell morphologies and the growth of neural cells. Our phage matrices, which could be easily functionalized with various ligands and growth factors, can be used as convenient test beds for investigating the functions of various biochemical stimulants on numerous cell types.
Novel phage-based therapeutics:
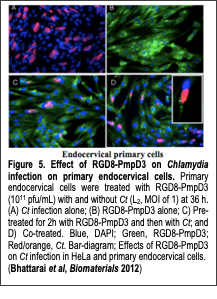
The primary goal of this research is to understand how specific sequences of protein/peptide and their display can affect the interaction between proteins and cells to develop novel phage-based Text Box: Figure 5. Effect of RGD8-PmpD3 on Chlamydia infection on primary endocervical cells. Primary endocervical cells were treated with RGD8-PmpD3 (1011 pfu/mL) with and without Ct (L2, MOI of 1) at 36 h. (A) Ct infection alone; (B) RGD8-PmpD3 alone; C) Pre-treated for 2h with RGD8-PmpD3 and then with Ct; and D) Co-treated. Blue, DAPI; Green, RGD8-PmpD3; Red/orange, Ct. Bar-diagram; Effects of RGD8-PmpD3 on Ct infection in HeLa and primary endocervical cells. (Bhattarai et al, Biomaterials 2012) therapeutics. An M13 phage possesses the unique ability to present multiple peptides or proteins on its surfaces, which makes it useful for various therapeutic purposes. We observed that phage displaying RGD peptides could be internalized into cells through integrin-mediated endocytosis. Furthermore, we enhanced the internalization process of the phage particles by engineering the phage with a high copy number (up to ~140 copies) of cyclic RGD peptides on their major coat proteins (Choi et al, Bioconj. Chem. 2014). This suggests that the phage could be used as therapeutic delivery vehicles. For example, we collaborated with Dr. Deborah Dean (Oakland Children’s Hospital, UC San Francisco) to develop a novel phage to use as a topical mucosal treatment or as a microbicide to prevent and ameliorate Chlamydia trachomatis (Ct) urogenital infections. No vaccines or microbicides are currently available to prevent sexually transmitted infections (STI) due to Ct. Ct is a Gram-negative obligate intracellular bacterium and the most common cause of bacterial sexually transmitted diseases (STD) worldwide. In our recent work (Bhattarai et al, Biomaterials 2012), we constructed phages that were engineered to express RGD (integrin binding) peptides and Ct polymorphic membrane protein D (PmpD), which is known to interfere with Ct propagation. The resulting phage significantly decreased Ct infections in HeLa cells and primary endocervical cells compared with those cells exposed to Ct alone (Fig. 5). Recently, we expanded our therapeutic research to cancer targeting based on collagen dystrophy. Collagens are over-expressed in various human cancers and subsequently degraded and denatured by proteolytic enzymes, which makes them a novel target for diagnostics and therapeutics. Genetically engineered bacteriophage (phage) is a promising candidate for the development of imaging or therapeutic materials for cancer collagen targeting due to its promising structural features. In our recent work (Jin et at, Biomaterials 2014), we genetically engineered M13 phages with two functional peptides, collagen mimetic peptide and streptavidin binding peptide, on their minor and major coat proteins, respectively. The resulting engineered phage functions as a therapeutic or imaging material to target degraded and denatured collagens in cancerous tissues. We demonstrated that the engineered phages are able to target and label denatured or cancerous collagens expressed on A549 human lung adenocarcinoma cells after conjugation with streptavidin-linked fluorescent agents. Our engineered collagen binding phage could be a useful platform for cancerous collagen imaging and drug delivery in various collagen-related diseases. Furthermore, we constructed the collagen-mimetic peptide library on the phage and screened against cancerous collagen tissues. We plan on further enhancing their specificity depending on collagen type and structures in future research.
M13-AAV Hybrid Phage for Drug Delivery and Tissue Engineering:
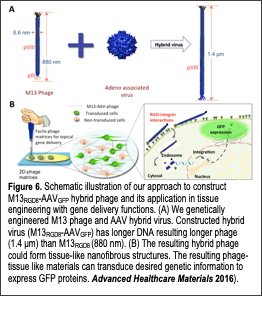
We developed a novel hybrid virus by combining phage genome and adeno-associated virus genome for novel drug delivery vehicle and tissue engineering materials (Advanced Healthcare Materials 2016). Recent advances in the phage biotechnology provide remarkable pathways to develop novel biomaterials. Phages possess many desirable features that make them attractive as versatile gene delivery materials. Phage have little harmful effects and are easily removed from the body through lysosomal degradation processes, causing few known side-effects. Phage can be modified to display functional peptide motifs on their minor (pIII, pIX) and major (pVIII) coat Text Box: Figure 6. Schematic illustration of our approach to construct M13RGD8-AAVGFP hybrid phage and its application in tissue engineering with gene delivery functions. (A) We genetically engineered M13 phage and AAV hybrid virus. Constructed hybrid virus (M13RGD8-AAVGFP) has longer DNA resulting longer phage (1.4 μm) than M13RGD8 (880 nm). (B) The resulting hybrid phage could form tissue-like nanofibrous structures. The resulting phage-tissue like materials can transduce desired genetic information to express GFP proteins. Advanced Healthcare Materials 2016). proteins. Large quantities of identical phage building blocks can be easily prepared through bacterial amplification. Due to their long-filamentous shape (aspect ratio: 130), phage can self-assemble into nanofibrous tissue-like matrix structures. Recently, we engineered various biochemical cues (i.e., RGD, IKVAV, and DGEA) on major coat proteins (pVIII) of the phage and developed self-assembled tissue engineering materials for regulating cellular behaviors of desired cells (Fig. 6). Using this phage-based tissue matrix system, we demonstrated that engineered phages could regulate various cellular behaviors such as proliferation and differentiation. We also demonstrated a novel tissue engineering materials with gene delivery functions through the hybridization of M13 bacteriophage (phage) and adeno-associate viruses (AAV). We engineered the M13 phage with RGD peptide on the major coat proteins. We then fused its gene with adeno-associate virus (AAV) gene with eukaryotic invading functional gene, inverse terminal repeat (ITR). The resulting M13-AAV hybrid phage exhibited enhanced internalization into target cells as compared to minor coat engineered phage with RGD or wild type phage. The resulting hybrid phage formed nanofibrous matrices that could support the cellular growth and deliver desired gene information into the target tissues. Our novel M13-AAV hybrid phage matrices can provide selective, stable, and safe gene delivery. We believe that it can be also developed as a topical therapeutic tissue patch in the future.
Collagen-Like Nanofiber Tissue Engineering Materials:
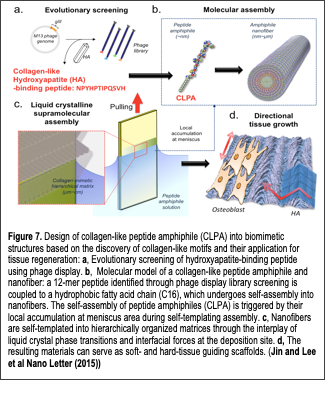
We developed a novel collagen-like nanofibril materials (Jin et al, Nano Letters 2015) to regenerate hard tissues. diagnosis/targeted drug delivery, and biosensors. Text Box: Figure 7. Design of collagen-like peptide amphiphile (CLPA) into biomimetic structures based on the discovery of collagen-like motifs and their application for tissue regeneration: a, Evolutionary screening of hydroxyapatite-binding peptide using phage display. b, Molecular model of a collagen-like peptide amphiphile and nanofiber: a 12-mer peptide identified through phage display library screening is coupled to a hydrophobic fatty acid chain (C16), which undergoes self-assembly into nanofibers. The self-assembly of peptide amphiphiles (CLPA) is triggered by their local accumulation at meniscus area during self-templating assembly. c, Nanofibers are self-templated into hierarchically organized matrices through the interplay of liquid crystal phase transitions and interfacial forces at the deposition site. d, The resulting materials can serve as soft- and hard-tissue guiding scaffolds. (Jin and Lee et al Nano Letter (2015)) In our previous work (Chung et al, Langmuir 2011), we identified a new class of 12-residue peptide that could bind to single crystal (100) hydroxyapatite (HAP) surfaces. Interestingly, the sequence responsible for HAP binding resembled the tripeptide repeat (Gly-Pro-Hyp) of type I collagen (see the sequence in Fig. 7). This peptide was able to template the nucleation and growth of crystalline HAP minerals in a sequence- and composition-dependent manner. Therefore, there is increasing interest for developing collagen-mimetic building blocks and their self-assembly through controlling the liquid crystal flow and the subsequent deposition of such building blocks to create biomimetic liquid crystal flow patterns over large areas. In this context, the recent discovery of directed self-assembly process termed “self-templating assembly”, which emulates nature’s self-assembly of incoming chiral molecules through the control of thermodynamic, kinetic and environmental factors, has opened new avenues to control liquid crystalline behaviors and the deposition of nanofibrous building blocks for the creation of various hierarchical structures, as demonstrated using the M13 bacteriophage as a model molecule. Therefore, expanding the self-templating assembly to other supramolecular and self-assembly system will be a great logical next step to develop hierarchically organized functional biomaterials. Recently, we reported the synthesis and assembly of collagen-like peptide amphiphiles into functional hierarchical, supramolecular structures. We designed a collagen-like peptide amphiphile (CLPA) through the conjugation between the previously identified collagen-like hydroxyapatite binding peptide together with hydrophobic alkyl chain (Fig. 7). The resulting CLPA forms nanofibers of uniform diameter through molecular self-assembly. This nanofiber-forming peptide amphiphile exhibits lyotropic liquid crystalline behavior in solution. We exploited the self-templating approach to create diverse hierarchical architectures analogous to structures often found in collagenous tissues (i.e., bones, cartilage, and tendons). The structural evolution of the self-templated architectures depends on initial CLPA concentration and the rate of deposition, which impose both thermodynamic and kinetic factors during the self-templating process. The resulting CLPA-based materials direct cellular-oriented growth and serve as scaffolds for biomineralization, showing their potential for soft and hard tissue engineering. To our knowledge, this work represents the first report describing the self-assembly of peptide amphiphiles into hierarchical collagen-like liquid crystalline surface coatings that are effective over large areas, and which may contribute to developing biomimetic materials and understanding bio-macromolecular assembly in in vivo systems.