Protein-Protein Interfaces
The primary goal of this research is to understand the mechanisms nature uses to create diverse hierarchical structures and exquisite functions in a spatially and temporally controlled manner from a simple nanofibrous building block through protein-protein assembly processes.
Biomimetic self-templating supramolecular structures:
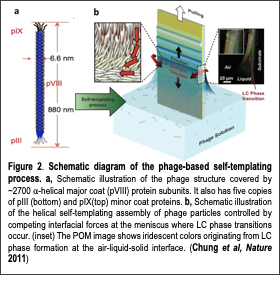
In nature, helical macromolecules such as collagen, chitin and cellulose are critical to the morphogenesis and functionality of various hierarchically structured materials. During tissue formation, these chiral fibrous macromolecules are secreted and undergo self-templating assembly, a process whereby multiple kinetic factors influence the assembly of the incoming building blocks to produce non-equilibrium structures. A single macromolecule can form diverse functional structures when self-templated under different conditions. Collagen type I, for instance, forms transparent corneal tissues from orthogonally aligned nematic fibers, distinctively colored skin tissues from cholesteric phase fiber bundles, and mineralized tissues from hierarchically organized fibers (Fig. 1). Nature’s self-templated materials surpass the functional and structural complexity achievable by current top-down and bottom-up fabrication methods. However, self-templating has not been thoroughly explored for engineering synthetic materials. In our recent paper (Chung et al, Nature 2011), we used phage-based nanofibers to determine the mechanism by which the hierarchical organization of self-assembled structures occurs. We used the M13 phage as a model system because it possesses collagen-like structural features with genetic flexibility (a helical nanofiber-like shape, monodispersity, and the ability to display functional peptides on its surfaces). A single-step process, which we called ‘self-templating assembly’, produces long-range-ordered, supramolecular structures showing multiple levels of hierarchical organization and helical twist (Fig. 2). Using our novel self-templating material assembly process, we could create many biologically induced hierarchical structures in a controlled manner. More importantly, we could create structures never observed in nature nor produced by other engineering approaches. Three examples of those distinctly newly created phases of supramolecular structures are reported in our work (Nature 2011): nematic orthogonal twists, cholesteric helical ribbons and smectic helicoidal nanofilaments. Both chiral liquid crystalline phase transitions and competing interfacial forces at the interface are found to be critical factors in determining the morphology of the templated structures during assembly. The resulting materials show distinctive optical and photonic properties, functioning as chiral reflector/filters and structural color matrices. In addition, M13 phages with genetically incorporated bioactive peptide ligands direct both soft and hard tissue growth in a hierarchically organized manner (See ‘Protein-Cellular Interfaces’ section for further discussion). Our newly developed phage-based material approach may provide a means for understanding the helical self-assembly of the hierarchical structures of biomaterials and give insight into hierarchical structure-function relationships in nature. Currently, we are applying our self-assembly techniques to other biomacromolecules (collagen, chitin and cellulose) and investigating their molecular assembly processes for the development of biomimetic hierarchical structures for tissue regeneration. Our newly develop phage-based material system was highlighted in President Obama’s US Congress report for the National Science Foundation (NSF) Budget entitled “Manufacturing goes viral” this year (2014) (Fig. 1). NSF produced a 30-min TV interview program based on this research and broadcasted it for general public education.
Butterfly Inspired Self-Templating Supramolecular Structures:
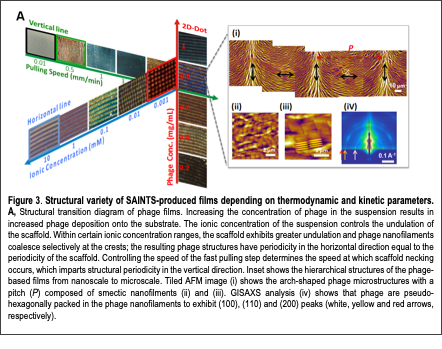
Recently, we developed a novel self-assembly process inspired by the butterfly morphogenesis process. Many natural biomaterials have exquisite physical, optical, and mechanical properties that scientists strive to replicate. These features are often attributable to hierarchical ordering: organized structure at the nano, micro, and larger length scales. Some examples include butterfly wings, beetle exocuticles, diatom skeletons, and mantis shrimp hammers. Achieving similar complexity by bottom-up self-assembly in the laboratory is highly desirable because it would enable advanced materials to be synthesized at large scales, with low cost and little labor. Recent advances in material engineering have self-assembled small molecules, polymers, and particles into functional optical, mechanical, and electrical structures and devices. In addition, techniques that exploit some of nature’s strategies such as inducing chiral liquid crystalline organization and self-templating assembly have achieved a degree of hierarchical ordering with desirable properties. However, limited control over the temporal and spatial resolution during self-assembly has limited the structural complexity, material properties, and range of non-equilibrium structures achievable. Recently, we developed a biomimetic fabrication Text Box: Figure 3. Structural variety of SAINTS-produced films depending on thermodynamic and kinetic parameters. A, Structural transition diagram of phage films. Increasing the concentration of phage in the suspension results in increased phage deposition onto the substrate. The ionic concentration of the suspension controls the undulation of the scaffold. Within certain ionic concentration ranges, the scaffold exhibits greater undulation and phage nanofilaments coalesce selectively at the crests; the resulting phage structures have periodicity in the horizontal direction equal to the periodicity of the scaffold. Controlling the speed of the fast pulling step determines the speed at which scaffold necking occurs, which imparts structural periodicity in the vertical direction. Inset shows the hierarchical structures of the phage-based films from nanoscale to microscale. Tiled AFM image (i) shows the arch-shaped phage microstructures with a pitch (P) composed of smectic nanofilments (ii) and (iii). GISAXS analysis (iv) shows that phage are pseudo-hexagonally packed in the phage nanofilaments to exhibit (100), (110) and (200) peaks (white, yellow and red arrows, respectively). process coined “Self-Assembly IN Transient Scaffolds (SAINTS)” that was inspired by butterfly wing morphogenesis (Fig. 3). During metamorphosis of wing scales, the structural organization and sites of chitin deposition are dictated by actin filament-based scaffolds. The actin filaments are subsequently disassembled after chitin assembly and thus serve as “transient scaffolds”. Inspired by the interplay between actin scaffolds and chitin fibrils, the SAINTS process exploits tunable menisci and M13 bacteriophage (phage) as transient scaffolds and nanofibers, respectively. During the SAINTS process, we controlled kinetic and thermodynamic parameters in order to vary the morphology and micro-spacing of menisci. These menisci served as scaffolds for the local accumulation, alignment, and deposition of phage nanofibers into ordered nano to macrostructures (Fig. 3). SAINTS-derived phage structures acted as butterfly wing-like tunable structural colors with optical switching behaviors, and as mechanically robust composite materials. Furthermore, SAINTS-induced micro-patterned phage arrays exhibited significantly improved piezoelectric performance.