Protein-Organic Material Interfaces
The primary goal of this research is to interrogate protein-organic material interfaces to understand how proteins bind desired chemicals through molecular recognition and to utilize it for the development of sensitive and selective biosensors.
Development of peptide based recognition element for biosensor:
Miniaturized smart sensors that can perform sensitive, selective, and real-time monitoring of explosives and biological toxins are tremendously valuable to our nation's ability to deploy effective homeland security measures and protect civilians and our military forces throughout the world. Current sensing devices are still far from being able to offer selective, sensitive, and real-time point-detection. They are also lacking in multi-analyte assessment, ease-of-use, and low manufacturing costs. To address these critical issues, we have developed a new approach, whereby the principles of molecular recognition in biology are mimicked to achieve highly selective binding to small molecular targets such as explosives and biotoxins. We discovered molecular recognition elements (MREs) against explosives (TNT and DNT) and biological and environmental toxins (Cholera toxin, PBDEs, and pesticides) by using directed evolution of phage peptide libraries. Using these MREs, we developed multiple nanocoatings for cantilever and quartz crystal microbalance sensing platforms which showed highly selective and sensitive multimodal detection (Jaworski et al, Langmuir 2008 and Anal. Chem. 2009) through the collaboration with Professors Arun Majumdar and Roya Maboudian (UC Berkeley). Using nuclear magnetic resonance spectroscopy, we studied the molecular level binding mechanism between the identified peptide (Trp-His-Trp) and desired target TNT chemicals (Jaworski et al, Langmuir 2011). In addition, we have recently developed a novel selective and sensitive biomimetic nanocoating by combining TNT receptors bound to conjugated polydiacetylene (PDA) polymers. PDA is a lipid-like polymer comprised of a conjugated polymer backbone with carboxylic acid and alkyl side-chains. The amphiphilic nature of PDA monomers facilitates its formation into supramolecular assemblies such as vesicles and membranes. PDA's conjugated polymer backbone can serve as a stable and sensitive colorimetric sensor due to changes in its conjugated electronic band structure resulting from interactions between target analytes and specific functional motifs on PDA's head-groups. In our recent work (Jaworski et al, Langmuir 2011), we described our colorimetric PDA-based TNT sensor development. Furthermore, we applied these PDA-TNT receptor coupled nanocoating materials into CNT-FET devices through collaboration with Professor Seunghun Hong (Physics, Seoul National University). Our recent paper (Kim et al, ACS Nano 2011) reported that selective binding events between the TNT molecules and phage display-derived TNT receptors were effectively transduced to sensitive SWNT-FET conductance sensors through the PDA coating layers. The resulting sensors exhibited unprecedented 1 fM sensitivity toward TNT in real time, with excellent selectivity over various similar aromatic compounds. Our biomimetic receptor coating approach may be useful for the development of sensitive and selective micro- and nanoelectronic sensor devices for various other target analytes.
Turkey skin collagen inspired colorimetric biosensors:
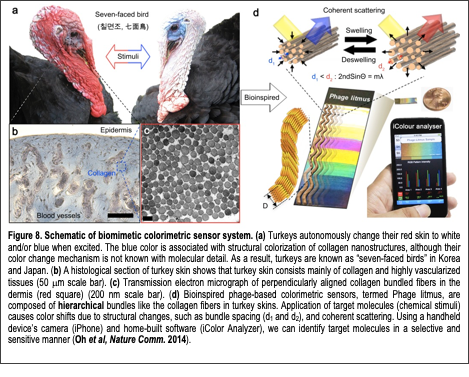
The primary goal of this research is to combine our understanding of protein-organic and protein-protein interfaces and develop a bio-inspired novel biosensor to detect desired chemicals in a sensitive and selective manner. Many animals change their skin colors to communicate, to express mood, for camouflage, or to respond to environmental changes. In the tissues of these animals, various nano and microscale components play roles in generating distinct colors and achieving rapid color changes. Inspired by nature, sensors that change color in response to target chemicals are being developed by employing biomimetic structures and mechanisms. In particular, structurally colored biomaterials, such as butterfly wings, beetle exocuticles, cephalopod skins, mammalian skins, and avian skins/feathers provide insight into developing colorimetric sensors. These materials exhibit brilliant colors that are derived from their hierarchically organized structures and are resistant to photobleaching. Furthermore, they can rapidly shift colors upon exposure to chemical vapors due to structural and/or refractive index changes. Therefore, both structurally colored materials in nature and their synthetic analogues are being explored as simple and portable colorimetric sensor platforms. A significant drawback of previous structural color sensors is their limited intrinsic affinity for specific targets of interest (e.g., explosives and pathogens) and resulting poor selectivity against analytes with similar chemical structures. Current methods to promote target specificity by either chemically incorporating specific recognition motifs or by synthesizing arrays of cross responsive platforms for “artificial nose” type pattern recognition are promising, but incorporating analyte-responsive elements into the sensing devices is still challenging because it requires complex designs and multistep synthetic pathways. Furthermore, many structurally colored sensors exhibit viewing-angle dependent color changes (iridescence) that may complicate analysis.
In our recent work (Oh et al, Nature Comm 2014), we demonstrated a novel biomimetic colorimetric sensing material composed of filamentous bacterial viruses (M13 phage; Fig. 8). Tunable colorimetric phage-based structures are fabricated using the self-templating assembly process we previously developed (Chung et al, Nature 2011). The resulting films are composed of quasi-ordered phage bundle nanostructures and exhibit viewing-angle independent colors due to the isotropic deposition of the phage nanobundles. These films mimic the structure of turkey skins (M. gallopavo), which are structurally colored blue due to the coherent scattering of light from collagen bundle-based nanostructures (Fig. 8a-c). Arrays of differently colored phage matrices, termed Phage litmus (Fig. 8d), rapidly swell or shrink upon exposure to external chemicals, resulting in color changes similar to those seen on turkeys when they get flustered (Fig. 8a). The chemicals are identifiable through color pattern analyses in a quantitative manner. To enhance selectivity, a trinitrotoluene (TNT)-binding motif identified by phage display is incorporated onto the phage coats. The TNT-binding phage litmus detects TNT down to Text Box: Figure 8. Schematic of biomimetic colorimetric sensor system. (a) Turkeys autonomously change their red skin to white and/or blue when excited. The blue color is associated with structural colorization of collagen nanostructures, although their color change mechanism is not known with molecular detail. As a result, turkeys are known as “seven-faced birds” in Korea and Japan. (b) A histological section of turkey skin shows that turkey skin consists mainly of collagen and highly vascularized tissues (50 mm scale bar). (c) Transmission electron micrograph of perpendicularly aligned collagen bundled fibers in the dermis (red square) (200 nm scale bar). (d) Bioinspired phage-based colorimetric sensors, termed Phage litmus, are composed of hierarchical bundles like the collagen fibers in turkey skins. Application of target molecules (chemical stimuli) causes color shifts due to structural changes, such as bundle spacing (d1 and d¬2), and coherent scattering. Using a handheld device’s camera (iPhone) and home-built software (iColor Analyzer), we can identify target molecules in a selective and sensitive manner (Oh et al, Nature Comm. 2014). 300 ppb with the aid of a common handheld device (iPhone) and can distinguish between similar nitroaromatic molecules (i.e. TNT, dinitrotoluene (DNT), and mononitrotoluene (MNT)). The facile synthesis, ease of use, portability, and successful introduction of tunable receptors suggest that Phage litmus colorimetric sensors can be useful for the detection of a wide variety of harmful toxicants and pathogens to protect human health and national security. Currently, we are developing the phage-litmus to enhance their sensitivity and selectivity for the environmental toxicants (PBDE, flame retardant). In addition, we also developed a phage to detect the acetone metabolite to monitor glucose levels for diabetic patients (data not shown). This foundational biosensing technology received first place in the “Berkeley Big Idea” business competition (2014). This technology was spun-off to the company ‘Bioinspira Inc’.