Protein-Electric Interfaces
The primary goal of this research is to interrogate protein structures and electric dipole relationships for the development of clean and green electric energy from the bio-inspiration of collagen-based piezoelectric properties in our body.
Biopiezoelectric energy generation:
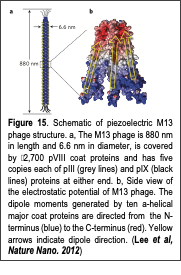

Piezoelectric materials are an attractive means of producing renewable and clean energy by utilizing ubiquitous vibrational and mechanical energy sources. The piezoelectric effect can be defined as the interconversion between mechanical and electrical energies induced by charge redistribution and separation upon the application of a mechanical or electrical stimulus to materials that lack inversion symmetry. Various inorganic piezoelectric materials exhibit strong piezoelectric properties, and have been used to generate electrical energy through commonplace activities, such as walking. Recently, several nanomaterials have been used to efficiently produce electrical energy by scavenging vibrational energy enough to operate small electronic devices. In addition, aligned, electrospun organic nanofibers have exhibited piezoelectric energy conversion efficiencies an order of magnitude higher than those Text Box: Figure 15. Schematic of piezoelectric M13 phage structure. a, The M13 phage is 880 nm in length and 6.6 nm in diameter, is covered by 2,700 pVIII coat proteins and has five copies each of pIII (grey lines) and pIX (black lines) proteins at either end. b, Side view of the electrostatic potential of M13 phage. The dipole moments generated by ten a-helical major coat proteins are directed from the N-terminus (blue) to the C-terminus (red). Yellow arrows indicate dipole direction. (Lee et al, Nature Nano. 2012) of bulk films. Although previous piezoelectric materials have provided useful schemes for energy generation, they are often made using environmentally harmful chemicals and/or energy intensive conditions, e.g., high temperatures, high electric fields, and extreme-pressures. In contrast, nature produces diverse, hierarchically organized functional materials at near-ambient temperatures using benign and abundantly available resources. These materials, which span in size from the nano to macroscale, include viral particles, abalone shells, teeth and bones. Interestingly, the piezoelectricity of biomaterials has been associated with critical physiological activities such as bone growth and remodeling. Such biopiezoelectricity occurs because biological building blocks (nucleotides and amino acids), biopolymers (DNA and polypeptides), and their higher-order assemblies lack inversion symmetry. Although biopiezoelectric materials pervade natural systems and present advantages over synthetic materials, they have not been utilized for engineered systems. This is mainly due to a lack of cost effective fabrication methods and little understanding of how to control biopiezoelectric properties at the molecular level.
In our recent work (Lee et al, Nature Nanotechnology, 2012), we fabricated novel bioinspired piezoelectric materials by exploiting the naturally aligned dipole structure of a bacterial virus, M13 phage. Structurally, M13 phage possess piezoelectric material features. The M13 phage has a long rod-like shape, 880 nm in length and 6.6 nm in diameter (Fig. 16a). Each phage is covered by 2,700 copies of a major coat protein (pVIII). The pVIII proteins have an α-helical structure with a dipole moment directed from the amino- to the carboxy-terminal direction and cover the body of the phage with five-fold rotational and two-fold screw symmetry (Fig. 16b). Because the M13 aligned protein coat structure lacks inversion symmetry, we hypothesized that the phage will possess intrinsic piezoelectric properties. Therefore, we first characterized the piezoelectric property of the M13 phage and developed a virus-based electric energy generator. Using piezoresponse force microscopy techniques through the Text Box: Figure 16. The first virus-based piezoelectric device that can operate a LCD display panel to show “1” by tapping the virus thin film device (Lee et al, Nature Nanotech. 2012). collaboration with Dr. Ramesh group, we characterized the structure-dependent piezoelectric properties of the phage at the molecular level. We discovered that the M13 phage possess axial and lateral piezoelectric properties. Their intrinsic piezoelectric response is 0.30 pm/V. Through the genetic engineering of the M13 phage, we enhanced the piezoelectric properties of the phage upto 0.70 pm/V. We also discovered that through the multi-layered films, we could enhance the piezoelectric properties up to 7pm/V. In 2012, we successfully demonstrated that a phage-based piezoelectric device could produce up to 6 nA and 400 mV which is enough to operate an LCD display panel (Fig. 16). More recently, we further improved the phage dipole strength and its self-assembly process in order to enhance the efficiency of the energy conversion of the phage-piezoelectric materials upto 100 nA and 1 V level (Manuscript is currently under preparation). Our phage-based piezoelectric material presents an adaptable and cost-effective means of harvesting energy from the environment and is an important step toward accessing the largely untapped potential of piezoelectric biomaterials.
The newly developed biopiezoelectric technology has attracted a lot of attention. Our virus electric energy generation was chosen as one of the 17 Biggest Scientific Breakthroughs of 2012 by iOS9. Scientific American chose this energy generating technology as one of top five future nanomanufacturing technologies, and it was also awarded R&D 100 award in 2013 as well.
Virus-based triboelectricity:
Triboelectricity is a phenomenon that generates electric potentials upon contact and separation between different materials. Recently, we report the triboelectricity of viral particles upon contact with metals and other materials (Kim et al, Nano Lett, 2021). We exploit the bacterial virus (M13 phage) as a programmable biomaterial to create precisely defined chemical and physical structures and characterize their contact-induced triboelectric properties. We genetically engineer one- to four-glutamate fused on the N-terminal of the major coat proteins of M13 phage and create a monolayer and multi-layer of the phage films. We then study their contact-induced triboelectric potential generation. Upon contact with Ti/Pt coated atomic force microscopy (AFM) tip, the phages can generate charges in a range of 2-4 μC m-2. Using Kelvin probe force microscopy (KPFM), we observe that more negatively charged phages can generate higher triboelectric potential and diffuse the charges faster than that of less negatively charged phages. Relative polarity comparison of the engineered phages and computational simulation results confirm that the more engineered glutamates we introduce, the lower the LUMO energy level becomes. Therefore, they can easily accept electrons from counter materials upon contact. We also demonstrate that the phage triboelectric nanogenerators (P-TENG) can produce ~76 V of voltage and ~5.1 μA of current, enough to power 30 light-emitting diode devices upon application of mechanical forces. Our results show that viral particles can induce triboelectric potential upon contact. Our triboelectric characterization of viral particles can be useful to harvest ubiquitous mechanical vibrational energy from environment and can provide a novel modality to detect desired viruses in the future.